By Kumudini Hettiarachchi
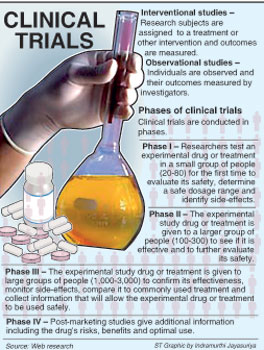
Clinical trials are experiments conducted on human beings to test the efficacy and safety of new drugs or devices or new indications of drugs or devices already in use. Explaining the process, Prof. Lal Jayakody, Professor of Pharmacology of the Medical Faculty, University of Colombo, said firstly such drugs are tested on animals and later on humans. These drugs are developed by the pharmaceutical industry and the development process is long drawn out and expensive as well as requiring the services of many experts.
“Therefore, only multinational pharmaceutical companies can afford to carry out such research and so far Sri Lanka has participated in small trials,” he said. (See box and graphic for how clinical trials are approved now)
Referring to the Clinical Trials Bill, Prof. Jayakody who pointed out that existing legislation has only about one or two sentences with regard to such research and these are “grossly inadequate”, and stressed that stringent legislation in this regard is “good in principle”.
The fact that must never be forgotten is that these are experiments on humans and their safety should be ensured as far as possible, the Sunday Times learns. Prof. Jayakody echoed the view that there should not be any secrecy around the Bill and urged for sufficient time to peruse the final draft and suggest amendments.
When asked whether the Clinical Trials Regulatory Division (CTRD) should be a stand-alone body, Prof. Jayakody pointed out that as most clinical trials pertain to drugs and devices, the focal point should be the Cosmetic, Devices and Drugs Authority (CDDA) and the CTRD should be under it.
The introduction of well-regulated clinical trials would be in keeping with global and regional trends, according to Prof. Jayakody and the “positives” include not only the development of expertise in researchers but also networks with other universities and agencies. While there would also be technological transfer to the country, the funds flowing in should only be considered a subsidiary benefit. “It is imperative that researchers safeguard the interests of participants and that research interests do not overwhelm the interests of participants,” he said.
Dealing with the “negatives”, this senior Professor issued a gentle reminder that when doctors agree to be investigators in clinical trials they should take up that responsibility fully, having weighed the pros and cons whether they could devote time amidst their busy schedules at hospitals and practice, for research is time-consuming.
Don’t fall victim to the lure of large amounts of money which come with clinical trials as well as the numerous trips abroad, he urges, adding that one should always be guided by ethical committees and institutional guidelines of universities.
“There must be a transparent policy. Payments must be declared and should be verifiable,” he said.
Pointing out that there are different kinds of health legislation in the pipeline, he underscored the fact that it was the National Medicinal Drugs Policy to regularize pharmaceuticals that the public has not only been awaiting earnestly but also clamouring for.
Prof. Jayakody was also concerned that many important committees had the same experts. This may be due to the lack of expertise or the difficulty faced by experts in the periphery to travel to Colombo.
“There is an urgent need to change this. The Colombo-centric people are also over-taxed. We need new blood, inputs from the second level who should be empowered and trained,” he said. Taking up the issue of conflict of interest, he said that merely making a declaration and leaving the room is not good enough. Those with such conflicts should leave decision-making committees altogether.
Meanwhile, the newly-inducted Sri Lanka Medical Association President Prof. Vajira H.W. Dissanayake said that they were awaiting the modified draft of the Clinical Trials Bill. “Clinical trials are part of the process by which new medicines, better and more effective than what we have, are being developed and brought into clinical practice. Therefore, as users of medicine one could say that people should in fact make some contribution towards that process because we benefit from medicines tried and tested on other people and found to be effective and are in clinical practice,” he said.
Adding a word of caution, however, he pointed out that in the use of any molecule as a therapeutic or food, there is a potential risk. Therefore, evaluation of these molecules should happen in a strictly-controlled and regulated environment.
There should be national legislation governing clinical trials because in its absence anybody can do anything, as whatever process is in place doesn’t have the force of law backing it, he pointed out.
The CTRD should come under the CDDA, he stressed, adding that “nowhere in the world is it separate from the drug regulatory authority”.
Explaining the three main components of the proposed legislation as regulation, process of ethics review and registration of the trials, Prof. Dissanayake said the drug regulatory authority would have the power to look at the investigation process of the research; recognised Ethics Review Committees (ERCs) would take up the second task and the registration of such trials would put the information in the public domain.
The SLMA has an ERC to provide ethical review for any clinical trial applications from institutions which do not have their own ERC, while there are eight more ERCs accredited by the CDDA comprising seven at the medical faculties and one at the Medical Research Institute. The ERC of the Colombo Medical Faculty has also got international recognition under WHO’s Strategic Initiative for Developing Capacity in Ethical Review (SIDCER), the Sunday Times learns.
Prof. Dissanayake added that when clinical trials are registered it is in the public domain and people will be aware of the opportunities available for experimental drugs. They could then seek help in cases of cancer or genetic disorders where there are no known cures. The SLMA’s Clinical Trial Registry had been recognised by the WHO in 2006 as a primary registry even before the one in India.
The process of conducting clinical trials
Currently clinical trials are being monitored by the Health Ministry’s 17-member Sub-committee on Clinical Trials set up in January 2009 chaired by Cosmetic, Devices and Drugs Authority Director Dr. Hemantha Beneragama.
In 2011, the sub-committee which includes experts from the Health Ministry, medical faculties, the Sri Lanka Medical Association, the Post-Graduate Institute of Medicine and the Medical Research Institute, dealt with 12 applications, the Sunday Times learns.
Eight applications have been approved, one rejected and three are pending. Before the sub-committee was set up there was no formal process. Now a special application along with a detailed protocol has to be submitted, with clear guidelines set out on the CDDA’s website (www.cdda.gov.lk), Dr. Beneragama said.
Some of the important criteria, according to Dr. Beneragama, are that the Principal Investigator in the research should be a local medical specialist; ethical approval should have been obtained from one of the eight ERCs recognised by the CDDA; and once the research is approved it should be registered at a WHO-recognised Clinical Registry, the sole registry currently being at the SLMA.
Another condition is that the research should be part of a multi-centre trial with one centre being in a reference country such as the USA, England, Australia, New Zealand, Canada or Singapore where there is good regulation.
When the clinical trial is launched reports have to be submitted once in six months, adverse effects informed immediately, if the trial is terminated that too should be informed immediately and if completed the end-of-trial report submitted, it is learnt. “We must promote research in the country, as drugs specific for Asians could be produced. Sri Lanka is well-suited for such research because our doctors are well-qualified and the country has a good mix of both infectious and non-communicable diseases.
The clinical trials initiative will also promote research and curb the brain drain,” Dr. Beneragama said.
Pointing out the role of the drug regulatory authority, he said it safeguards the health of the people and prevents their exploitation while facilitating high-quality research carried out in conformity with the National Safety Regulations.
Concern over Lankans being used for drug research would lead to abuse
Laws to use Sri Lankans for drug research will be made public shortly, a Health Ministry official assured.
This came in the wake of protests against the proposed Clinical Trials Bill that will see Sri Lankans being used for experiments.
The final draft is not ready yet. As soon as the Legal Draftsman sends it, the Health Ministry will circulate it among the experts and the public, Ministry Secretary Dr. Ravindra Ruberu told the Sunday Times.
Experts, however, were disturbed over the “final synopsis” of the draft circulated among them earlier and alleged that if the Clinical Trials Regulatory Division (CTRD) is de-linked from the drug regulatory authority -- the Cosmetic, Devices and Drugs Authority (CDDA) -- serious issues could crop up.
“Then those with vested interests can easily influence matters with regard to clinical trials, endangering the men, women and children who would be ‘subjects’ or ‘participants’ in such research,” a source said, pointing out that fears were mounting as the final draft had not been seen even by those who were part of the National Steering Committee for Clinical Trials set up in 2009.
Another source wondered whether provision had been made in the Bill for a legal framework for Ethics Review Committees (ERCs) and their functioning.
ERCs are a vital cog in the wheel of holding clinical trials as ethical review is mandatory, the Sunday Times understands. The other matter of concern among many was whether the authorities are looking at clinical trials as a money-making venture for the government’s coffers. This may lead to the exploitation of the participants in such research, with money overriding the safety of people, they said.
The Sunday Times learns that the first draft of the Clinical Trials Bill was developed after numerous meetings of the National Steering Committee convened by the Department of National Planning under the Finance Ministry and chaired by Finance Secretary P.B. Jayasundera.
The consensus among all those whom the Sunday Times spoke to was that a Clinical Trials Act based on internationally-accepted ‘Good Clinical Practice (GCP)’ Guidelines is essential and should be geared to safeguard those participating in clinical trials. A Bill once finalised by the Legal Draftsman is taken before the Cabinet by the relevant minister for approval and then presented to Parliament.
http://www.sundaytimes.lk/120129/News/nws_42.html
No comments:
Post a Comment